

Molecular: AgNO3 (aq) + KCl (aq) AgCl (s) + KNO3 (aq) 2. When hydrochloric acid (chemical formula: HCl) reacts with sodium carbonate (chemical formula: Na2CO3), one of two reactions will take place, depending on the relative … The balanced ionic equation for the reaction is Na 2 CO 3 + 2AgNO 3 - > 2Na 2 NO 3 + Ag 2 CO 3. His students will be investigating precipitation reactions, just … 2AgNO 3 + Na 2 CO 3 → Ag 2 CO 3 + 2NaNO 3 Silver (I) nitrate react with sodium carbonate to produce silver (I) carbonate and sodium nitrate. Describe what double-replacement reactions are used for. 2 AgNO3 + Na2CO3 = Ag2CO3 ↓ + 2 NaNO3 It is also used as an oxidizing agent.For each of the following reactions, determine what the products of each reaction will be. Identify all of the phases in your answer. 2 AgNO3(aq) + Na2CO3(aq) → Ag2CO3(s) + 2 NaNO3(aq) Since Silver carbonate has a very low solubility in water (0.031 g/L (15 ☌)), it will precipitate as a white solid. (c) Oxidation-Reduction: Ca(s) has an oxidation number of 0, while Ca2+(aq) has an oxidation number of +2-hence Ca is being oxidized. There are two common techniques for balancing redox equations: oxidation number change method ion-electron method (also called the half-reaction method). The silver oxide precipitates out of solution as a brown solid, while the sodium nitrate remains in aqueous solution. When hydrochloric acid (chemical formula: HCl) reacts with sodium carbonate (chemical formula: Na2CO3), one of two reactions will take place, depending on the relative quantities of each … Down. T F The oxidation state of nitrogen changes from +6 to +2. into If you add Na2CO3 drop-wise into HCl and mix evenly, which means that HCl is exceeded, the reaction is: Na2CO3 + 2HCl = 2NaCl + H2O + CO2 (g). After decomposition, the Na2CO3 had a mass of 1.57 grams. Silver nitrate and calcium chloride will react in a double displacement reaction (which.
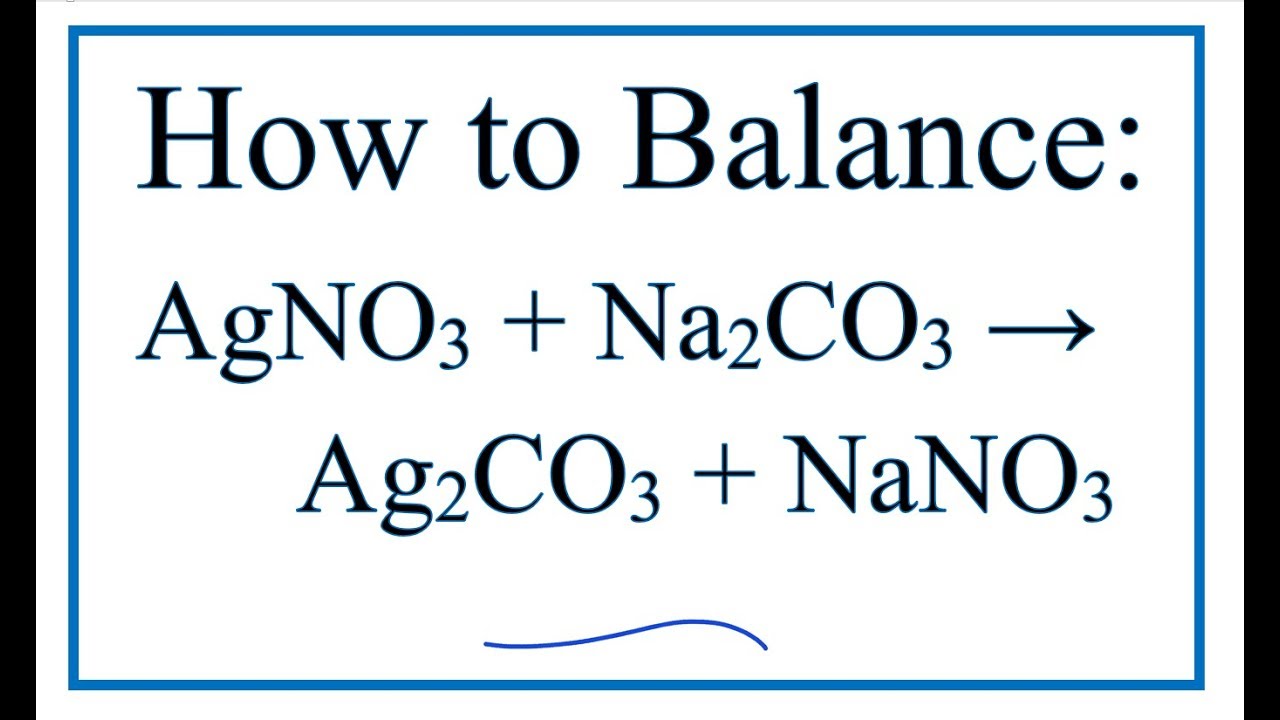
The answer will appear below Always use the upper case for the first character in the element name and the lower case for … I am assuming that you are reacting two aqueous solutions. What Is The Double Displacement Reaction For NH4OH+H2SO4? If I recall my first year chem class, that would be a double replacement reaction forming Silver Sulfate and Sodium Nitrate. Be sure to clearly label the tests and outcomes.Na2CO3.



 0 kommentar(er)
0 kommentar(er)
